02.04 The Mole and Conversion: Conversion Problem One—Text Version
Problem: If a sample of magnesium (Mg) atoms has a mass of 36.8 grams g, how many atoms are in the sample?
Step one–What are you looking for?
In this question, the given information is 36.8 grams of pure magnesium (Mg). The unit needed for the final answer is atoms Mg.
Step two–What do you already know?
Avogadro's Number: ![]()
Molar Mass of Mg (from periodic table): ![]()
Step three–Where does the information go?
Set up the problem, starting with the given measurement.
36.8 g Mg ×
Next, include the first conversion factor of fraction, which will cancel g Mg.
36.8 g Mg × ![]()
Lastly, include the last conversion factor of fraction, which will cancel mol Mg leaving atoms Mg.
36.8 g Mg × ![]() ×
× 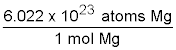
Step four–Solve it!
Multiply the top numbers and divide by the bottom numbers. Don't forget to include the unit in your answer.
36.8 × 6.022 × 1023 ÷ 24.305 ÷ 1 = 9.12 × 1023 atoms Mg